
These adult stem cells, which exist in the tissue for decades, serve to replace cells that are lost in the tissue as needed, such as the growth of new skin every day in humans.
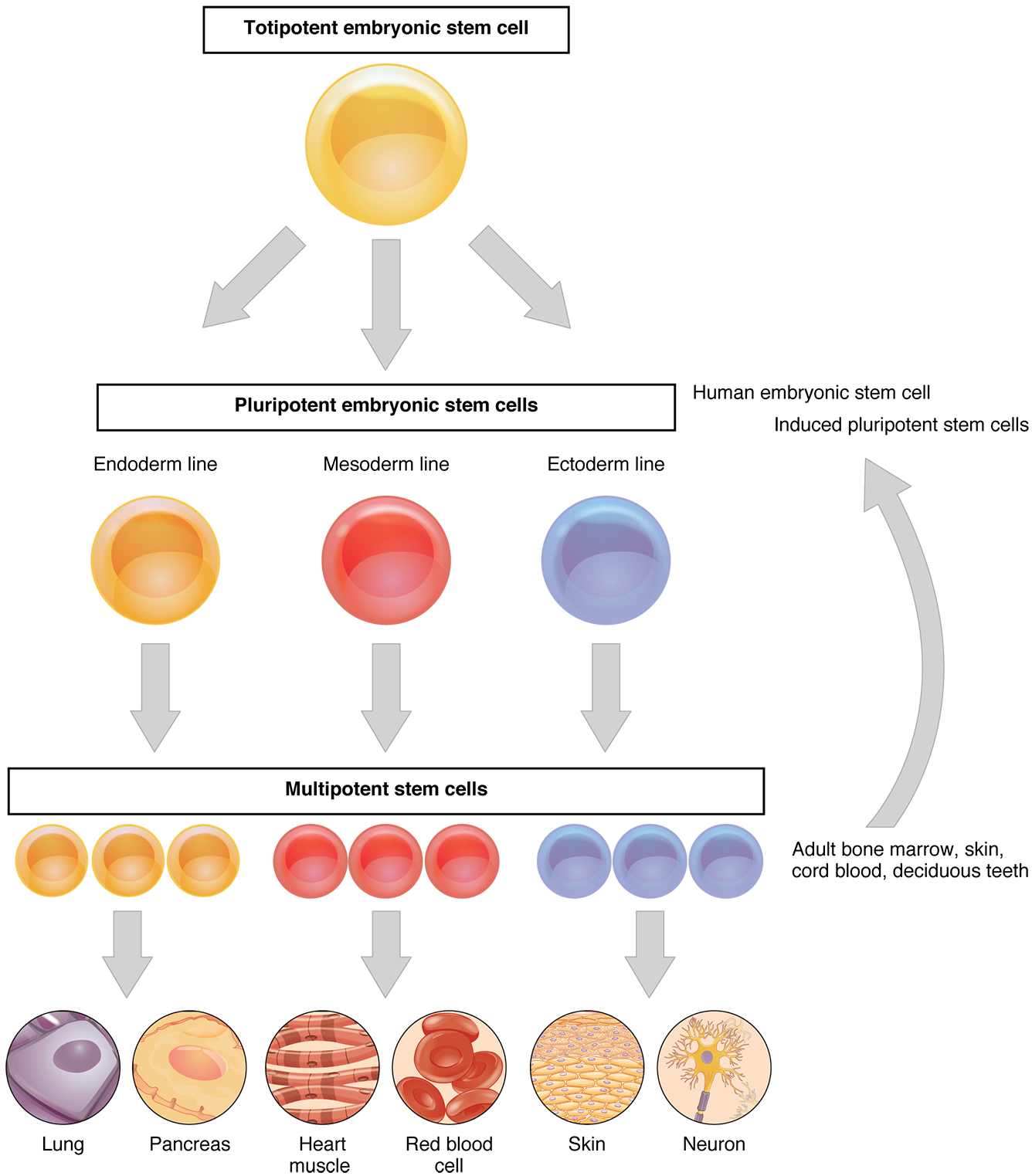
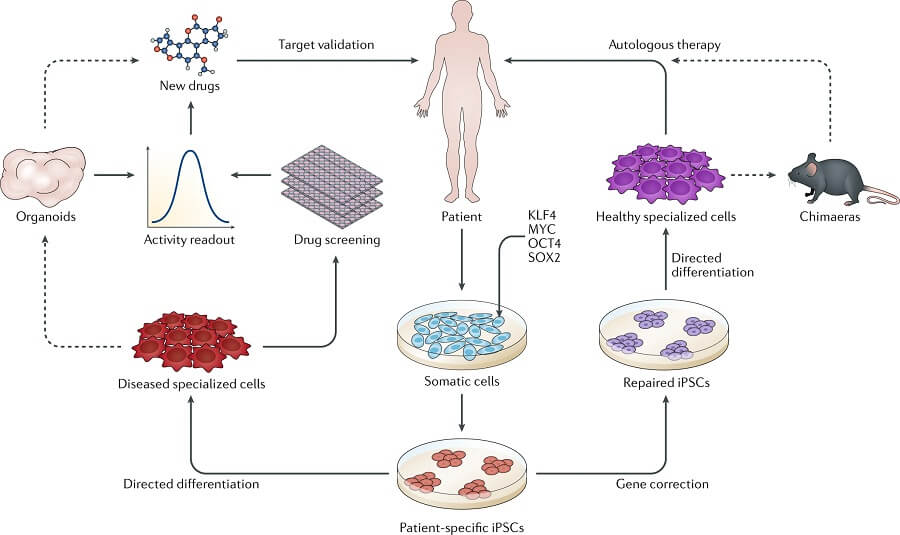
Unlike embryonic stem cells, which can become any cell in the body (called pluripotent), adult stem cells, which have been found in a wide range of tissues including skin, heart, brain, liver, and bone marrow are usually restricted to become any type of cell in the tissue or organ that they reside (called multipotent). The Agencies recognize that considerations around the ethical conduct of such research are complex and continually evolving, and welcome comments and discussion, and commit to the continued evolution of TCPS 2.Adult stem cells, also called somatic stem cells, are undifferentiated cells that are found in many different tissues throughout the body of nearly all organisms, including humans. In 2014, the guidelines were integrated into the 2nd Edition of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2). The Act also prohibits certain activities such as cloning humans or creating chimeras. The Act applies to the derivation of human pluripotent stem cells from human embryos, but does not apply to research using human embryonic stem cell lines that have already been derived. In March 2004, an Act Respecting Assisted Human Reproduction and Related Research, became law. The guidelines provided for the review of human stem cell research applications by a Stem Cell Oversight Committee (SCOC). Until then, Canada had no laws to govern human pluripotent stem cell research, nor were there any guidelines for researchers, research ethics boards, or funding agencies on how human pluripotent stem cells may be derived and used. In January 2002, after a year of discussion and consultation, the group produced a report to CIHR's Governing Council, which was unanimously accepted and formed the basis of human pluripotent stem cell research guidelines that were publicly announced in March 2002. Indeed the Working Group considered its mandate to cover all human pluripotent cells, whatever their source, and the final guidelines were worded with that consideration in mind. Although the source of induced pluripotent stem cells does not raise unique ethical concerns, there are other ethical issues around related to the experimental use of human pluripotent stem cells whether they are derived from embryos or adults. While research on human adult stem cells was not included in the Working Group's mandate, recent scientific research has confirmed the possibility of generating human pluripotent stem cells with properties similar to embryonic stem cells from adult cells (e.g., induced pluripotent stem cells). Its mandate was to advise CIHR as to whether human embryonic stem cell and human embryonic germ cell research should be considered eligible for CIHR funding. In recognition of this and because of the complex ethical issues that it raises, the President of CIHR convened the Ad Hoc Working Group on Stem Cell Research in the fall of 2000. At the same time, the derivation and use of human pluripotent stem cells raise ethical and social issues and legal concerns of interest to Canadians. Few other areas of science have generated as much excitement, scrutiny and controversy. Stem cell research has the potential to provide treatments for a host of debilitating diseases including Alzheimer's, Parkinson's, diabetes, multiple sclerosis, heart disease, and spinal cord injury. The Development of Guidance for Human Pluripotent Stem Cell Research in Canada


 0 kommentar(er)
0 kommentar(er)
